Li Tianhua, Wang Tao Department of Neurosurgery, Xuanwu Hospital October 6, 2024, 20:01 Beijing

Article link: https://doi.org/10.1136/jnis-2024-022189
Wang T, Luo J, Li T, et al. Stenting versus medical treatment alone for symptomatic intracranial artery stenosis: a preplanned pooled individual patient data analysis. J Neurointerv Surg. Published online August 15, 2024. doi:10.1136/jnis-2024-022189
Keywords
Symptomatic intracranial artery stenosis
PTAS
Ischemic stroke
Intracranial artery stenosis
01
Background
There is debate about whether the safety and efficacy of percutaneous transluminal angioplasty and stenting (PTAS) for symptomatic intracranial artery stenosis (ICAS) differs significantly from that of medical therapy alone. This study aimed to determine the safety and efficacy of the two treatments for symptomatic ICAS.
02
Key Points
The SAMMPRIS and VISSIT trials showed that PTAS was associated with a higher short- and long-term risk of stroke or death compared to medical therapy alone. However, the CASSISS trial showed no significant difference in short- and long-term stroke between PTAS and medical therapy alone. It is currently debated whether PTAS treatment for symptomatic ICAS is significantly different in terms of safety and efficacy from drug treatment alone.
This pre-planned individual patient data (IPD) meta-analysis included 400 participants treated with PTAS and 409 treated with drug treatment alone from two large multicenter randomized clinical trials (SAMMPRIS and CASSISS). The results showed that there was no significant difference between PTAS treatment and drug treatment alone in terms of long-term stroke or death, but the short-term stroke risk of PTAS was significantly higher than that of drug treatment alone. Race, hyperlipidemia, and the type of responsible event may have a potential impact on the safety and efficacy of PTAS.
This IPD analysis provides the highest level of evidence that drug treatment is still the best treatment, and PTAS cannot be recommended as the first-line treatment for patients with symptomatic ICAS. There is a trade-off between the risk of adverse events and revascularization, and patients considering PTAS treatment should be assessed rigorously.
03
Methods
This pre-planned IPD analysis included 400 patients treated with PTAS and 409 patients treated with drug monotherapy alone from the two large multicenter randomized clinical trials SAMMPRIS and CASSISS. The primary outcome was stroke or death within 30 days of enrolment or an ischemic stroke in the territory of the responsible artery after 30 days of enrolment.

Figure 1. Flow chart of the Study
04
Results
PTAS increases the risk of stroke/death in the short term and is therefore not recommended as the first-choice treatment for symptomatic ICAS. We need to find a balance between the risk of stroke and the benefit of revascularization. For symptomatic ICAS patients, if they are in the Western population, have hyperlipidemia or a history of TIA, they will face a higher risk of undergoing PTAS and should be more cautious before considering PTAS.
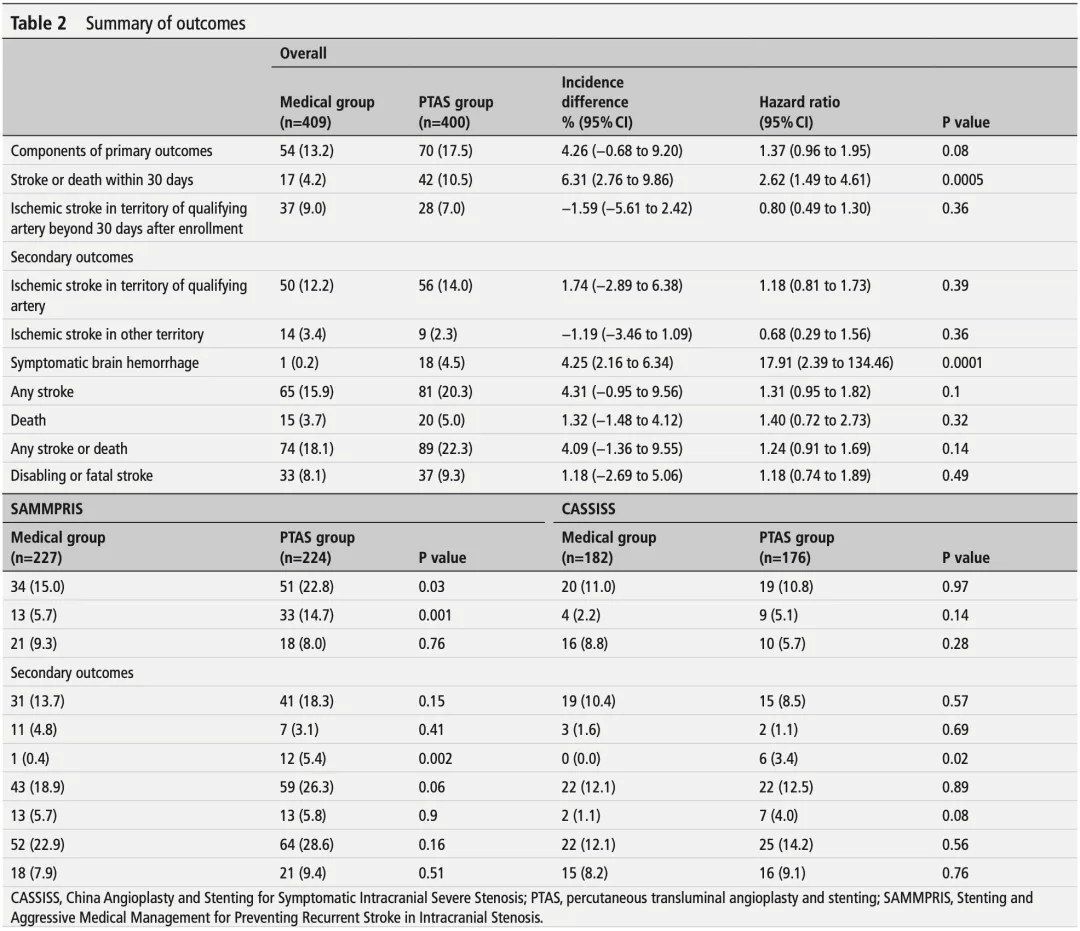
Table 2. Summary of outcomes
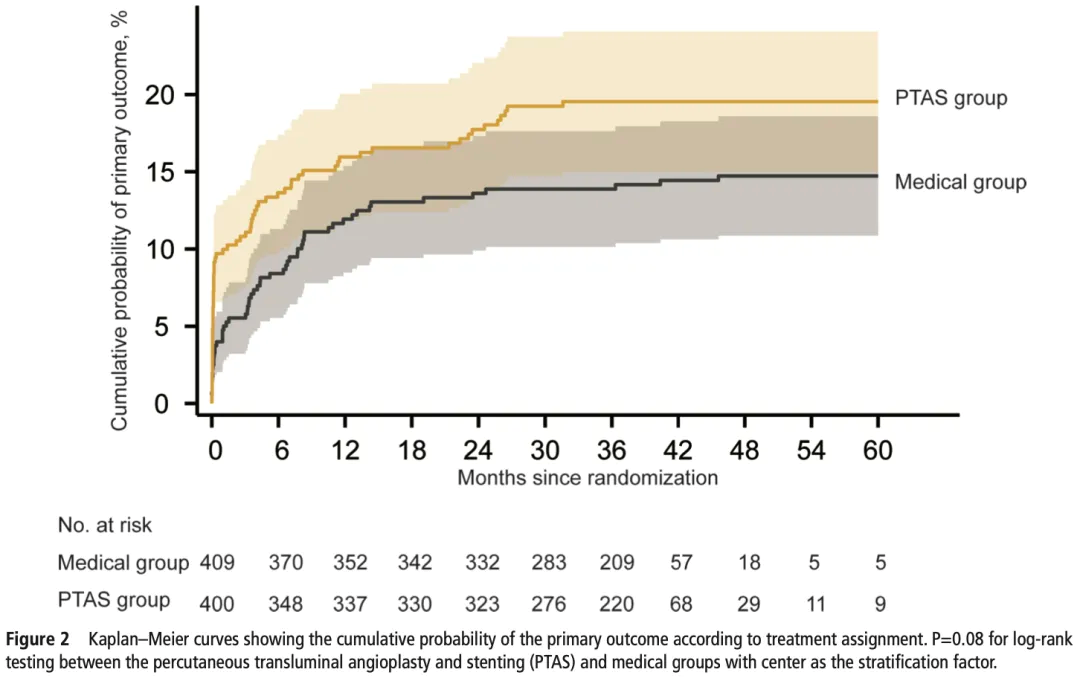
Figure 2. KM curves showing the cumulative probability of the primary outcome according to treatment assignment.
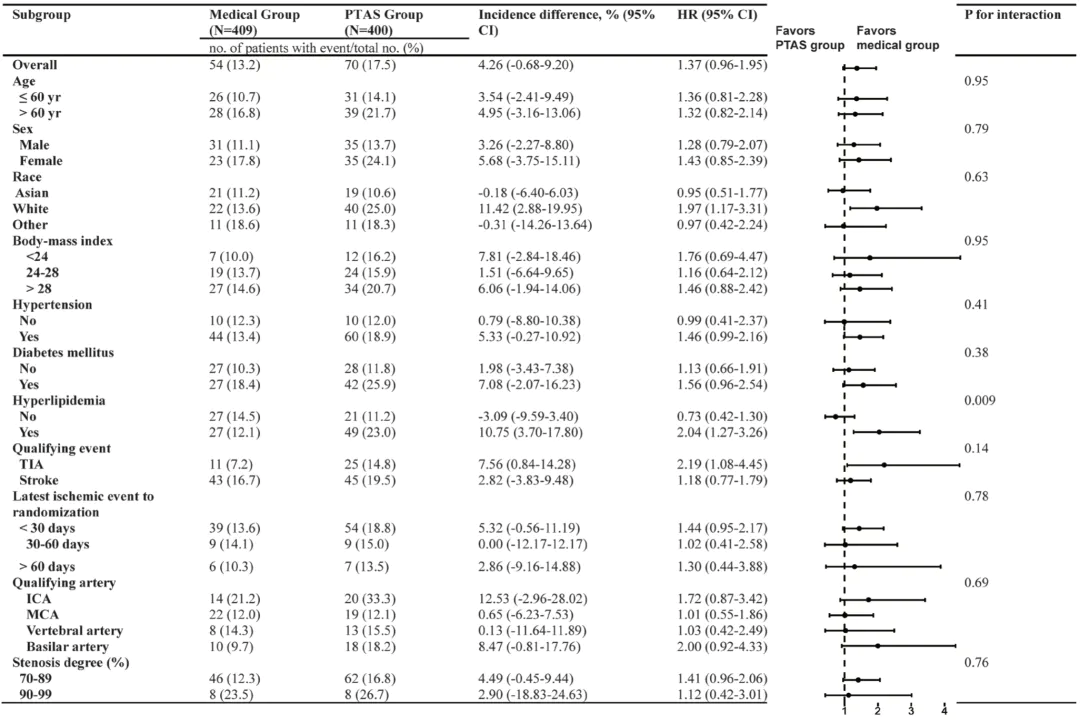
Figure 3. Subgroup analysis of the primary outcome Conclusion
05
Conclusion
PTAS increases the risk of stroke/death in the short term, and is therefore not recommended as the first-choice treatment for symptomatic ICAS. We need to find a balance between the risk of stroke and the benefit of revascularization. For patients with symptomatic ICAS, if they are in the Western population, have hyperlipidemia or a history of TIA, they will face a higher risk of PTAS and should be more cautious before considering PTAS.
Professor Wang Tao's comments
The three multicenter RCTs in the field of interventional treatment of intracranial artery stenosis comparing stent combined with drug therapy and drug therapy alone are the SAMMPRIS, VISSIT and CASSISS studies, with which everyone is familiar. We are very fortunate to have collaborated with Professor Colin P Derdeyn, Co-PI of SAMMPRIS, and used the original data from the SAMMPRIS and CASSISS studies to complete an IPD-meta analysis (meta-analysis based on individual patient data). The IPD-meta analysis is currently recognized as the highest level of evidence for studies, as it provides the highest level of evidence for the biggest controversy in the field of intracranial artery stenosis intervention treatment: stent combined drug therapy or simple drug therapy. There were no surprises in the results, as none of the three studies supported stent treatment.
So what is the significance of this study? First, although it is a generally accepted conclusion, it provides the highest level of evidence. Second, this study is just the beginning of a series of studies. Our team will conduct further subgroup analyses on hot topics in the field of interventional treatment of intracranial artery stenosis, such as the timing of surgery, population screening, and the degree of stenosis, as long as the data is feasible. Finally, the recent publication of BASIS confirmed that simple balloon dilatation may be an important direction for the future interventional treatment of intracranial artery stenosis. This series of IPD meta-analyses will also provide support for future population selection.
Author
Li Tianhua

A doctoral candidate in the Department of Neurosurgery of Xuanwu Hospital, Capital Medical University, under the supervision of Professor Jiao Liqun.
Research interests:
basic and clinical research on ischemic cerebrovascular disease.
Scientific research experience:
So far, he has published 8 articles as the first or co-first author in SCI journals such as International Journal of Surgery, Journal of Neurointerventional Surgery, European journal of neurology, and CNS Neuroscience & Therapeutics, as well as 1 article in a Chinese core journal.
Reviewing experts
Wang Tao

Attending Neurosurgeon, associate researcher, associate professor, master's supervisor, doctoral supervisor, graduated from Peking Union Medical College (Tsinghua University School of Medicine) with an eight-year clinical medicine degree.
He is committed to the diagnosis and treatment of surgical, interventional and combined operations for ischemic cerebrovascular diseases such as intracranial artery stenosis, carotid artery stenosis and Moyamoya disease. He is Beijing Science and Technology Rising Star, Youth Talent of Science and Technology Think Tank of the Department of Strategic Development of the China Association for Science and Technology, and Seed Talent of Xuanwu Hospital of Capital Medical University. So far, he has published more than 40 SCI papers as the first/corresponding author in journals such as JAMA, Stroke, Ageing Research Review, Aging and Disease, SVN, JNIS, Cochrane Reviews, etc., co-edited 2 books, and obtained 8 authorized patents. He has presided over 8 projects including the National Natural Science Foundation of China and the Beijing Natural Science Foundation. He is a young member of the editorial board of Brain Circulation and the Chinese Journal of Cerebrovascular Diseases. He is a member of the Beijing Medical Association's Young Physicians Branch of the Neurointerventional Specialty, a member of the Beijing Neuroscience Society's Neurointerventional Professional Committee, a standing member and deputy secretary general of the Chinese Medical Education Association's Stroke Revascularization Professional Committee, and a volunteer doctor in China.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "China-INI," "chinaini.org" are trademarks of China International Neuroscience Institute.
© 2008-2021 China International Neuroscience Institute (China-INI). All rights reserved.