Department of Neurosurgery, Xuanwu Hospital 2024-05-18 18:02 Beijing

The 10th European Stroke Organisation Conference 2024 (ESOC 2024) was held on May 15-17 in Basel, Switzerland.
Dr. Gao Peng, Director of the Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, delivered a lecture at the main session of the closing ceremony, entitled: Stenting Plus Medical Therapy vs. Medical Therapy Alone for Symptomatic Intracranial Atherosclerotic Stenosis: 7-Year Results of a Randomized Controlled Trial (CASSISS-FU).
Dr. Lu Xia and Dr. Liu Delin of our department were present at the same time to present the academic poster, sending first-time, first-person perspective panoramic academic reports as "frontline reporters".

Theme of the report
Stenting Plus Medical Therapy vs. Medical Therapy Alone for Symptomatic Intracranial Stenosis (CASSISS-FU): 7-Year Results of the CASSISS Trial.
Stenting Plus Medical Therapy VS Medical Therapy Alone for Symptomatic Intracranial Stenosis: 7-Year Results of the CASSISS Trial

Speaker
Gao Peng
Xuanwu Hospital, Capital Medical University

Dr. Gao Peng was speaking at the closing plenary session of ESOC 2024 in Basel, Switzerland.
Study Background
Symptomatic intracranial atherosclerotic stenosis (ICAS) poses a significant risk of ischemic stroke globally, accounting for 10% of all ischemic strokes in the United States and up to 50% in China. Previous randomized trials (SAMMPRIS, VISSIT, and CASSISS) have shown no significant benefit in patients with symptomatic, severe intracranial stenosis treated with stents in combination with medications. However, it remains unclear whether ICAS would benefit from stenting in terms of long-term follow-up.
Study Objectives
Previously, the CASSISS trial showed results of a 3-year analysis and has been published in 2022. The CASSISS-FU trial extended the follow-up window with the aim of clarifying whether the benefits gained in long-term prevention of stroke using stenting in combination with medication offset the perioperative risks of stenting compared with medication alone during a median of 7 years of follow-up.
Study Design
CASSISS was a multicenter, open-label, randomized trial conducted in 8 centers in China. We recruited patients with transient ischemic attack (TIA) or ischemic stroke (mRS score 0-2) due to severe intracranial atherosclerotic stenosis (ICAS) (stenosis of 70%-99%). Eligible patients were randomly assigned in a 1:1 ratio to either the stent combined with medication group or the medication alone group. The primary outcome was stroke or death within 30 days or ipsilateral ischemic stroke occurring beyond 30 days. Secondary outcomes included ipsilateral stroke, disabling or fatal stroke, and death after enrollment.
Inclusion and Exclusion Criteria
1. Exclusion of patients who had a TIA or ischemic stroke in the past 3 weeks (lasting more than 24 hours; confirmed by DWI)
Exclusion of patients who had experienced only brainstem or basal ganglia penetrating infarcts;
No loading doses of DAPT medications allowed.
Results of the Study
From March 2014 to November 2016, a total of 380 patients were enrolled and randomly assigned to the stenting group (188) and the medication group (192). Of these 380 patients, 358 (176: stent implantation group, 182: medication group) were identified as eligible and included in the FAS for final analysis (Figure 1). Of the 358 patients, 237 (66.2% completed) 7 years of follow-up.
The median follow-up was 7.4 years (IQR 6.0-8.0).
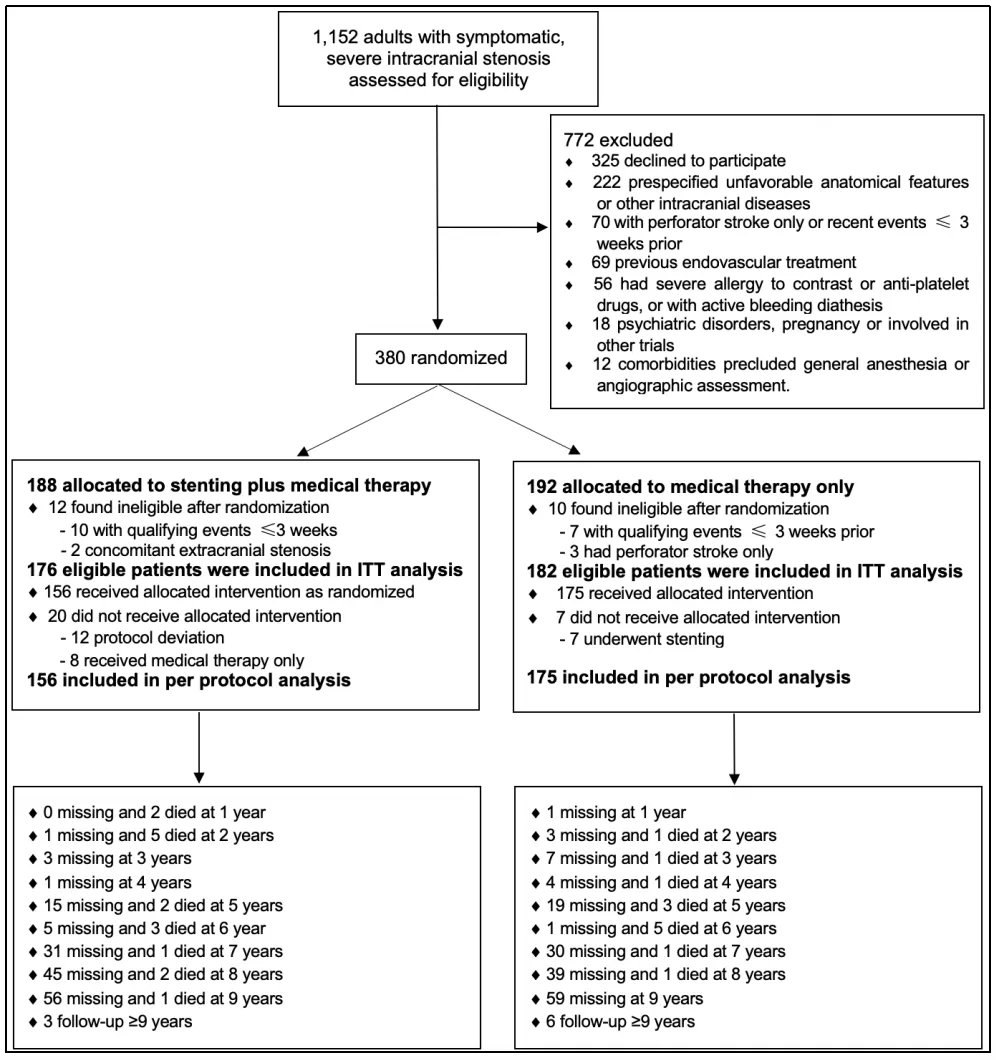 Figure 1 Flow chart of the study
Figure 1 Flow chart of the study
The primary outcome (composite event of stroke or death within 30 days or ipsilateral ischemic stroke after 30 days) showed no difference between the two groups during the 7-year follow-up period (HR, 1.02 [95% CI, 0.58-1.77]; P=0.97) (Figure 2).
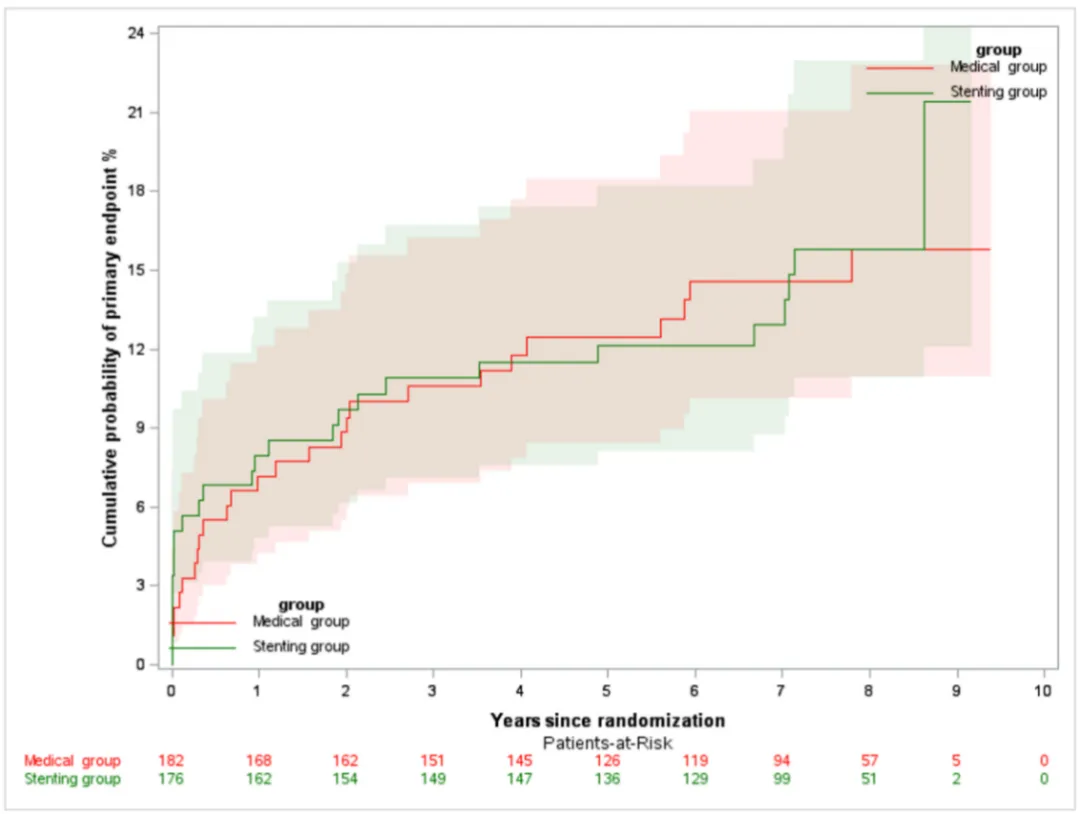 Figure 2 Primary outcome (composite event of stroke or death within 30 days or ipsilateral ischemic stroke after 30 days)
Figure 2 Primary outcome (composite event of stroke or death within 30 days or ipsilateral ischemic stroke after 30 days)
Study Conclusions
Over a follow-up period of up to 7 years, stenting combined with pharmacologic therapy in symptomatic patients with severe ICAS still fails to demonstrate long-term benefit under current patient selection criteria.
Potential Effects
Pharmacotherapy remains an important treatment for long-term stroke prevention in patients with symptomatic severe intracranial atherosclerotic stenosis.
Reviewer

Wang Tao
Xuanwu Hospital, Capital Medical University
Author

He Xiaoxin
Xuanwu Hospital, Capital Medical University
© Copyright
It is strictly prohibited for any media, website or individual to reprint or otherwise copy publish/publish the text, pictures, videos and other materials in this article without authorization. Media, websites or individuals who have been authorized by the agreement must indicate the source of the use, and violators will be held liable in accordance with the law.
△ Special Note
The text, pictures, audio and video contained in this page are for medical professionals only. The content contained on this page is only the personal opinion of experts and cannot replace the judgment of medical professionals. This page is not open to non-medical professionals, please understand.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "China-INI," "chinaini.org" are trademarks of China International Neuroscience Institute.
© 2008-2021 China International Neuroscience Institute (China-INI). All rights reserved.