Department of Neurosurgery, Xuanwu Hospital 2024-04-17 18:30 Beijing
On April 11, 2024, Prof. Chen Zhan's team from the Department of Neurosurgery, Xuanwu Hospital of Capital Medical University published a research paper entitled "Single-cell sequencing reveals VEGFR as a potential target for CAR-T cell therapy in chordoma" in the journal British Journal of Cancer (JCR Partition Q1, IF: 8.8). Dr. Wu Huantong and Dr. Zhang Boyan from Department of Neurosurgery, Xuanwu Hospital were the co-first authors, and Prof. Chen Zhan and Prof. Duan Wanru were the corresponding authors.

Chordoma is a rare primary bone tumor that originates from the residual tissue of the spinal cord. The annual incidence of chordoma is about 0.08 per 100,000, with a male predominance and a peak incidence around the age of 50 years. Currently, there is no effective treatment for chordoma, and complete surgical resection is the main treatment nowadays. Patients with incomplete resection due to surgical complications (e.g., adjacent nerves or blood vessels) are treated with postoperative radiation therapy. The median overall survival (OS) for patients with chordoma is about 6 to 7 years, but the clinical course varies. Chordomas generally present as moderately to poorly differentiated, locally aggressive malignant tumors, but they may present in a variety of ways, leading to rapid progression in some patients. As a result, approximately one-third of chordoma patients will develop localized spread and metastasis, and chordomas are less likely to be cured in the case of recurrent, refractory, or metastatic disease.
Therefore, effective therapies are urgently needed to minimize recurrence and progression, and to treat recurrence when it occurs.
Prof. Chen Zhan's team collects tumor sample tissues from clinical patients for single-cell sequencing, and searches for potential targets for chordoma treatment by analyzing tumor heterogeneity and gene expression in chordoma patients. In 2022, Prof. Chen Zhan's team published the first single-cell sequencing article on chordoma patients "Single-cell transcriptome profiling reveals intra-tumoral heterogeneity in human chordomas" published in Cancer Immunol Immunotherapy (JCR partition Q1, IF: 5.8).
Recently, the research team expanded the chordoma sequencing sample size on the basis of the previous study to detect the relevant expression of targets and signaling pathways in chordomas. The target VEGFR, which can be used for chordoma immunotherapy, was found and VEGFR CAR-T cells were constructed. Meanwhile,TGF-β signaling pathway was found to be a highly expressed intercellular signaling pathway in the tumor microenvironment of chordoma in the sample tissues of chordoma cells. Therefore, adding a TGF-β scFv structure to the VEGFR CAR structure can bind TGF-β in the tumor microenvironment and improve the effect of TGF-β on chordoma cells.
Single-cell sequencing was performed on tumor tissue samples from 14 chordoma patients. The assay revealed high heterogeneity in the chordoma tumor samples and complex cellular components in the tumor tissues. In terms of tumor cells, there were differences between primary and recurrent tumor samples. In the tumor microenvironment, increased expression of TGF-β in immune cells (mainly T and B cells) as well as increased expression of VEGFR2 in endothelial and tumor cells were found, which was consistent in both primary and recurrent samples. Immunohistochemistry of chordoma tumor samples further confirmed these expression patterns (Figure 1).
Next, we performed intercellular communication analysis to identify potential interaction pathways within the chordoma tumor microenvironment. Cellchat analysis revealed that TGF-β signaling within the TME was highly expressed. The main feature of this pathway is that T and B cells, as well as endothelial cells, are the main senders of TGF-β signaling, while myeloid and fibroblasts are the receivers. The most prominent ligand-receptor interaction in this pathway is TGFβ1-TGFβR1/TGFβR2 (Figure 2).
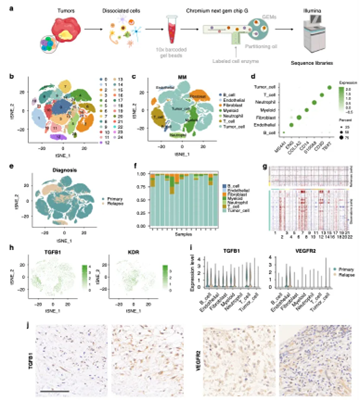
Figure 1. Single-cell heterogeneity profile of human chordoma tumors
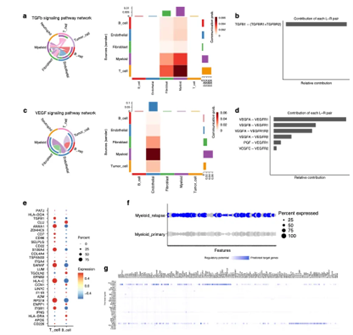
Figure 2. Analysis of cellular communication networks in the chordoma tumor microenvironment
After identifying the high expression of TGFβ and VEGFR in chordoma by sequencing, the group prepared two kinds of CARs targeting vascular endothelial growth factor receptor: the VEGFR CAR and the VEGFR/TGF-β scFv CAR bound to TGF-β scFv. Next, the activation and killing effects of CAR-T cells on VEGFR-expressing chordoma cells were evaluated using the JHC-7 chordoma cell line with malignant proliferative potential. Control, VEGFR and VEGFR/TGF-β scFv CAR-T cells were incubated with JHC-7 cells at different effector-target ratios. Both VEGFR CAR-T cells and VEGFR/TGF-β scFv CAR-T cells exhibited cytotoxicity against JHC-7 cells, which was positively correlated with the E/T ratio.
To assess the sustained effects of CAR-T cell variants on tumor cells, JHC-7 tumor cells were introduced into the culture system on days 0, 2, 4, and 6. The sustained killing effect of VEGFR and VEGFR/TGF-β scFv CAR-T cells on tumor cells was observed at different time intervals.
TGF-β plays a crucial role in immunosuppression of solid tumors. Therefore, we co-cultured VEGFR CAR-T cells and VEGFR/TGF-β CAR-T cells with JHC-7 cells at an E:T ratio of 4:1 for 20 h. Exogenous TGF-β factor was added to the culture system. VEGFR/TGF-β scFv CAR-T cells exhibited stronger cytotoxicity compared to the VEGFRCAR-T experimental group. The cytotoxicity of both CAR-T cells was decreased when co-cultured with TGF-β. However, VEGFR/TGF-β scFv CAR-T's reduced TGF-β within the culture system, which increased the killing effect. VEGFR/TGF-β scFv CAR-T cells showed better sustained killing ability (Figure 3).
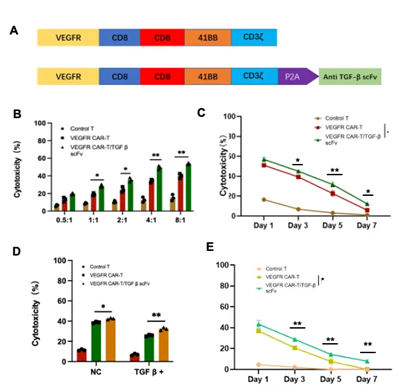
Figure 3. Preparation and composition of VEGFR CAR and VEGFR/TGF-β CAR-T cells
To evaluate the effectiveness of CAR-T cells in a clinical setting, we tested their killing effect in a patient-derived chordoma-like organ model expressing VEGFR. Control, VEGFR and VEGFR/TGF-β scFv CAR-T cells were incubated at different E/T ratios. VEGFR CAR-T cells and VEGFR/TGF-β scFv CAR-T cells exhibited significant cytotoxicity against organoids, and cytotoxicity was positively correlated with the E/T ratio. The cytotoxicity of VEGFR/TGF-β scFv CAR-T cells was superior to that of VEGFR CAR-T cells.
In order to assess the sustained cytotoxicity of CAR-T cells against primary chordoma cells, the group co-cultured CAR-T cells with primary chordoma cells, and primary tumor cells were repeatedly added at one-day intervals to assess the sustained cytotoxicity of CAR-T cells. It was found that VEGFR/TGF-β scFv CAR-T cells exhibited longer lasting sustained cytotoxicity against chordoma-like organs. The concentration of TGF-β in the cell supernatants in the co-culture system was also examined, and the VEGFR/TGF-β scFv CAR-T cells exhibited an increased affinity for TGF-β in the culture system, thus preserving the cytotoxicity of the CAR-T cells (Figure 4).

Figure 4. Killing effect of VEGFR/TGF-β in patient-derived chordoma-like organ models
The group analyzed single-cell sequencing data from 14 chordoma patients, thus providing important results for chordoma biology as well as malignant, mesenchymal, and immune cell mapping in chordomas. Sequencing results showed that VEGFR is a potential target for CAR-T treatment of chordoma and that TGF-β can be combined for chordoma immunotherapy. Experiments showed that VEGFR/TGF-β scFv CAR-T cells exhibited strong cytotoxicity against chordoma cells, including primary chordoma-like organs, and sustained killing of tumor cells. Although further preclinical validation of CAR-T cells in xenograft models or other effective in vivo models for chordoma patients is needed, this study provides a new therapeutic option for chordoma patients.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "China-INI," "chinaini.org" are trademarks of China International Neuroscience Institute.
© 2008-2021 China International Neuroscience Institute (China-INI). All rights reserved.