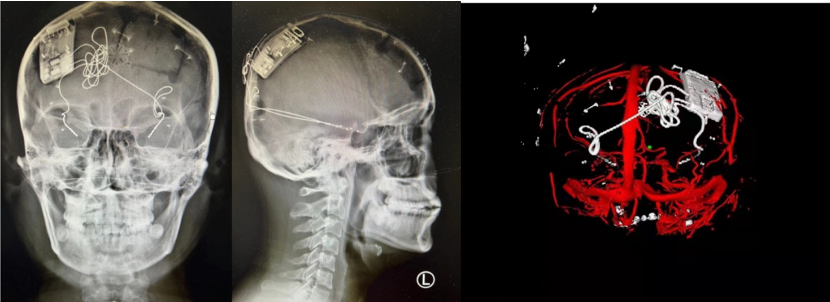
On March 9, 2022, the stereotaxic and functional brain disease team led by Professor Zhao Guoguang, President of Xuanwu Hospital of Capital Medical University, and Professor Shan Yongzhi of Neurosurgery, successfully treated a patient with refractory epilepsy (bilateral temporal lobe) with implantation of the closed-loop reactive neurostimulation system Epilcure™. The operation went smoothly, and all the indicators of the intraoperative stimulator worked normally. The postoperative reconstruction showed that the electrode position was accurate and the EEG signal was clear. The successful completion of this implantation operation marks the first patient enrollment in the Epilcure™ National Medical Products Administration (NMPA)-registered clinical trial led by Xuanwu Hospital.
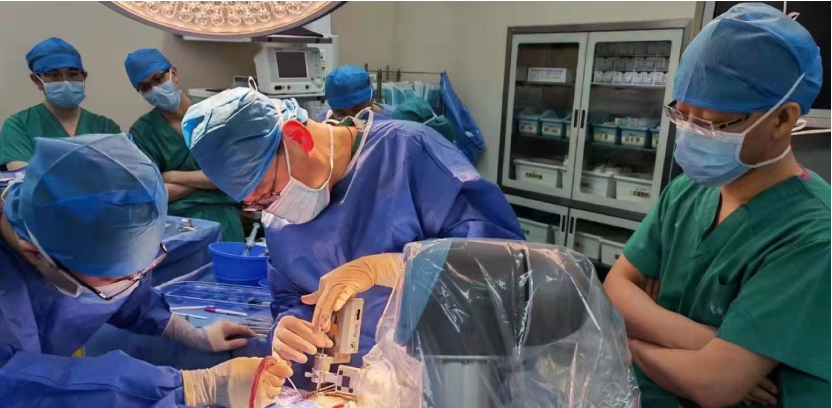
The patient was male and was diagnosed with bilateral temporal lobe epilepsy after admission. This type of epilepsy is a relative contraindication to craniotomy, and traditional treatment is not effective in the case of uncontrolled medication. The closed-loop reactive neurostimulation system is an important clinical application of "brain-computer interface". This technology uses artificial intelligence chips to be implanted in the skull, intracranial electrodes are implanted into the brain, and the EEG rhythm is monitored day and night without interruption. The occurrence of epilepsy, that is, the activation of exogenous interference rhythms, directly blocking the formation of epilepsy in the epileptogenic foci, and accurately controlling the activity of the loop. This new technology has brought good news to patients with epilepsy in multifocal or functional areas. The foreign product was approved by the US FDA in 2013, but it has been used as a "neck stuck" technology for long-term technical blockade and restrictions in China. The implantation of this system means that the "brain-computer interface" high-tech product with independent intellectual property rights has reached the final step before full-scale clinical use after the arduous efforts and research of the domestic clinical and basic research teams.
As the National Medical Center for Neurological Diseases, Xuanwu Hospital, under the leadership of Professor Zhao Guoguang, director of the center, started the research on "brain-computer interface" early in China. Perhaps many people still remember the opening ceremony scene of the 2014 World Cup in Brazil: a patient with complete spinal cord injury caused by a car accident, who had been paraplegic for 9 years, was able to stand, walk, and drive for the first time with the help of the "regain walking plan". Through the brain-computer interface technology, the patient gradually restores the motor function of the lower limbs, and can drive the exoskeleton robot to walk. On February 6, 2018, Professor Zhao Guoguang, cooperated with Professor Miguel Nicolelis, the initiator of the "Walking again" plan. Xuanwu Hospital became the first country in Asia to join the Walk again project (WAP). Based on this work, the center has established artificial intelligence brain network laboratory, brain-like intelligence clinical translation research center, Sino-US co-constructed brain connectomics laboratory, optogenetic neural circuit laboratory, etc. with the advances of artificial intelligence and brain connectomics research, Xuanwu Hospital strive to promote the "final step" of brain science to clinical translation.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "China-INI," "chinaini.org" are trademarks of China International Neuroscience Institute.
© 2008-2021 China International Neuroscience Institute (China-INI). All rights reserved.