INTRODUCTION
Brain metastases are a frequent complication in patients with melanoma. In the past, brain metastases almost invariably contributed to neurologic morbidity and death. However, the prognosis for many patients has been substantially improved by major advances in neuroimaging, improved options for the neurosurgical and radiotherapeutic management of brain metastases, improved management of metastatic disease at systemic sites, and demonstrated activity of systemic treatments against brain and other sites of central nervous system (CNS) metastases (eg, leptomeningeal, spinal cord).
The management of patients with melanoma and brain metastases will be reviewed here. General aspects of the clinical manifestations, diagnosis, and management of cancer-related brain metastases are discussed separately.
●Overview – Brain metastases are a frequent complication in patients with advanced regional and metastatic melanoma and are an important cause of both morbidity and mortality. The treatment of patients with melanoma brain metastases is rapidly evolving and should be distinguished from the current approach for patients with nonmelanoma brain metastases.
●Treatment approaches – Historically, locoregional treatments with surgical resection and/or radiation therapy (RT) were the only options to control brain metastases. However, newer systemic treatments such as immunotherapy and BRAF plus MEK inhibitors have intracranial efficacy and provide an alternative for patients with metastatic melanoma. As such, management of brain metastases benefits from a multidisciplinary management plan (algorithm 1).
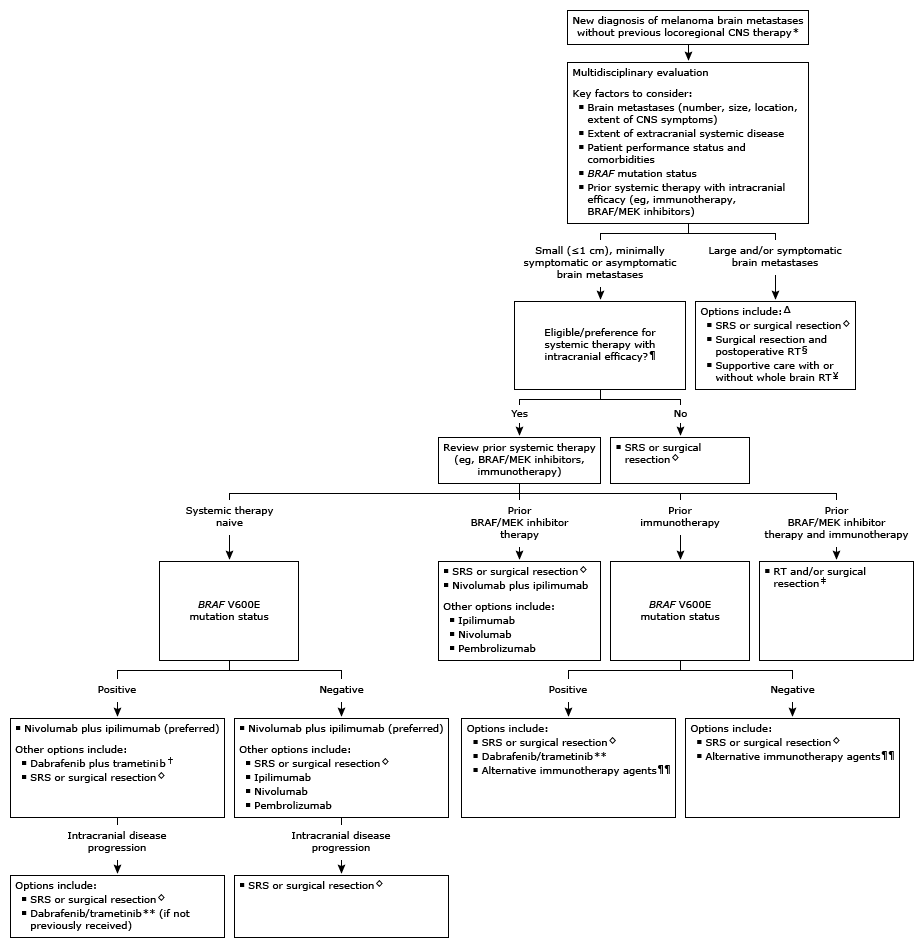
The approach to brain metastases assumes that the most likely diagnosis is metastatic melanoma, or that the diagnosis has been confirmed pathologically. However, not all new tumors or mass lesions in patients with melanoma are metastases, and alternative diagnoses should be considered before treating empirically, particularly for single masses and in the absence of systemic metastatic disease. Surgery may be indicated in such situations before proceeding with additional therapy, even for relatively small or asymptomatic tumors.
CNS: central nervous system; SRS: stereotactic radiosurgery; RT: radiation therapy; PD-1: programmed cell death receptor 1.
Locoregional CNS therapy includes surgical resection, SRS, and/or RT.
Optimal patients have limited comorbidities, appropriate performance status, and extracranial involvement.
Some patients may be candidates for systemic therapy following locoregional CNS therapy.
Patients with a single brain metastasis are candidates for surgical resection. SRS may be an option for patients who are not candidates for surgery, have multiple brain metastases, and/or have lesions that are surgically inaccessible or in eloquent areas.
Postoperative RT to the surgical cavity may be deferred in selected patients. Refer to UpToDate topics on melanoma brain metastases. Close monitoring for disease recurrence with periodic neuroimaging is appropriate regardless of treatment approach.This approach may be offered to patients with a short life expectancy, in whom systemic therapy is unlikely to offer substantial benefit, and/or large intracranial tumor burden.
In patients who have previously received prior immunotherapy and BRAF/MEK inhibitors, the choice and sequence of definitive CNS therapy depends primarily on the number, size, and location of brain metastases, as well as the extent of CNS symptoms and overall performance status.
Combined BRAF/MEK inhibition is an alternative option to combined immunotherapy in patients with BRAF V600 mutations requiring rapid extracranial disease response or in patients who are ineligible for immunotherapy.
If BRAF/MEK inhibition is offered as subsequent treatment after immunotherapy for progressive intracranial disease, use should be limited to patients with small and minimally symptomatic brain metastases.
For patients with prior exposure to a single-agent PD-1 inhibitor, some experts offer combination immunotherapy with nivolumab plus ipilimumab, although data are limited for this approach. Clinical trials evaluating immunotherapy in this setting are encouraged.
●Small (<1 cm), minimally symptomatic or asymptomatic brain metastases – For systemic therapy-naïve patients with small (<1 cm), minimally symptomatic or asymptomatic untreated brain metastases and extracranial disease burden, a closely monitored trial of systemic therapy with intracranial efficacy and deferral of locoregional central nervous system (CNS) therapy is an acceptable treatment approach. Selection of a specific regimen takes into account the nature and extent of extracranial disease, the presence or absence of a BRAF mutation, and patient performance status and comorbidities.
●Large and/or symptomatic brain metastases – For patients who are not eligible for systemic therapy due to prior therapies received or the large and symptomatic nature of the brain metastases, and those who progress on an initial trial of systemic therapy, locoregional CNS-directed therapy depends largely on the number and location of lesions.
●Single brain metastasis (>3 cm) – For patients with a single large (>3 cm) metastasis in an accessible location, we suggest surgical resection rather than radiation (Grade 2B). Lesions with diagnostic uncertainty, symptomatic edema, or posterior fossa location are especially important to resect, when possible. For patients who cannot undergo surgery, focal RT options for large tumors include hypofractionated stereotactic radiosurgery (SRS) and fractionated RT.
For patients who undergo incomplete resection, focal RT (SRS or fractionated) is typically indicated postoperatively to treat the residual tumor and cavity. For those who undergo complete surgical resection, some contributors routinely treat with RT to the resection cavity to maximize the chance of durable local control. Other contributors may forego postoperative RT when systemic immunotherapy is planned. Postoperative RT remains an option if targeted therapy or other forms of systemic therapy are planned, to reduce the risk of leptomeningeal disease. Close monitoring for disease recurrence with periodic neuroimaging is appropriate regardless of treatment approach.
●Multiple or larger brain metastases – For patients with multiple small brain metastases, all of which are amenable to SRS, we recommend SRS alone rather than SRS plus whole brain radiation therapy (WBRT) or WBRT alone (Grade 1B). The addition to WBRT after primary SRS may improve local control but has no overall survival (OS) benefit and increases neurocognitive impairment.
The timing of immunotherapy in relation to SRS is an evolving, active area of investigation. For patients receiving both SRS and immunotherapy, the most common approach is SRS followed sequentially by immunotherapy. However, some UpToDate experts have successfully used concurrent SRS and immunotherapy, which can achieve high levels of intracranial response and durable control of brain metastases with acceptable toxicity.
For larger metastases that are not amenable to single-fraction SRS, options may include hypofractionated SRS, fractionated focal RT, and surgical resection. Such treatment should be combined with systemic therapy to control extracranial disease.
●Patients with short life expectancy – For patients with a short life expectancy, in whom systemic therapy options are no longer likely to offer substantial benefit, and a large intracranial tumor burden, WBRT may be offered. In many cases, discussion of hospice is appropriate, especially when prognosis and quality of life are dismal.
●Surveillance – Patients with brain metastases remain at risk of CNS progression even after definitive treatment. As such, all patients should have imaging surveillance every two to three months with brain magnetic resonance imaging (MRI), or contrast-enhanced computed tomography (CT) if MRI is not possible.
●Duration of immunotherapy – Immunotherapy may be discontinued after at least six months of therapy if evaluation of extracranial disease burden shows a complete clinical response confirmed on two sequential imaging evaluations approximately three months apart, and brain lesions are either improving or stable over the same interval on serial neuroimaging without development of new metastases.
You can find professional doctors and experts about this disease here for your further consultation and treatment.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
A single copy of these materials may be reprinted for noncommercial personal use only. "China-INI," "chinaini.org" are trademarks of China International Neuroscience Institute.
© 2008-2021 China International Neuroscience Institute (China-INI). All rights reserved.